Background: As reported in our preliminary work (Blood (2022) 140 (Supplement 1): 9142-9143) myeloid neoplasms (MN) with MYC-positive double minutes (dmin) are mostly AML and often show a cytomorphological proximity to APL. We here now present detailed genotypical and phenotypical characteristics to address the question if MN with MYC dmin might represent a distinct hematologic entity.
Aim: In-depth characterization of cytomorphological, mutational, transcriptional and clinical features of 76 MN with MYC dmin and analysis of the amplified chromosomal region and its effect on gene expression (GE).
Methods: We analyzed 60 bone marrow (BM) and 16 peripheral blood (PB) samples of 76 pts with MN and MYC dmin (36 female, 40 male; median age 75 yrs, range 44-89 yrs). The diagnosis was established following WHO guidelines. Dmin and MYC amplification were assessed by chromosome banding analysis and FISH. Cytomorphologic examination included assessment of APL-like features, i.e. high number of atypical hypergranulated promyelocytes, high number of Auer rods, faggot(-like) cells and pseudo Chediak-Higashi (PCH) granules. Molecular genetic analyses comprised a targeted NGS panel (all pts, median coverage 1500x), WGS (16 pts, median coverage 100x) and WTS (40 pts, 50 Mio reads). Mut frequencies and GE levels were compared to AML cases without MYC dmin (“non dmin” AML). Survival data were available for 41 pts (median follow-up: 12 months).
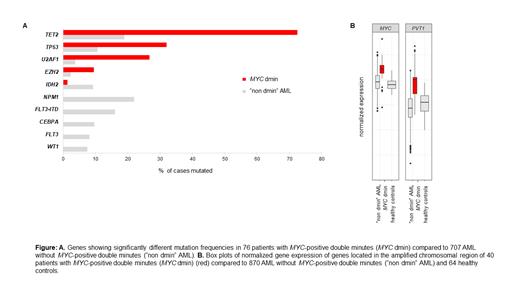
Results: According to WHO 2022 most of the 76 cases were AML-MR (myelodysplasia-related) (55/76, 72 %, 9 of these post MDS or post MDS/MPN). Three (4 %) cases each were classified as AML with maturation or without maturation, while one case with AML was not further classifiable due to insufficient material quality and lack of defining markers. Other diagnoses comprised MDS with biallelic TP53 inactivation (4/76, 5 %), MDS-IB1 (1/76, 1 %), CMML-2 (4/76, 5 %), CMML-1 (1/76, 1 %), MDS/MPN (3/76, 4 %) and MPN in blast phase (1/76, 1 %). BM blast count was ≥10 % in 52/60 (87 %) pts, likely underestimated in the remaining 8 samples due to lack of particles, and all 16 pts where only PB was available showed >2 % blasts. Out of 50 pts with fully assessable cytomorphology 48 (96 %) presented with a highly dysplastic granulopoiesis, often including severe dysplasia in other myeloid lineages, independent of myelodysplasia-related genetic markers. Twenty-nine of 50 (58 %) pts were APL-like (≥2 APL-like features), but with a higher degree of maturation than APL. A complex karyotype (ck, ≥3 aberrations in addition to MYC dmin) was present in 25/76 pts (33 %), whereas 21/76 (28 %) pts presented with MYC dmin as the only cytogenetic aberration. TET2 mut, which represented the most frequent mut in MYC dmin pts (55/76, 72 %), often were biallelic events (36/55 pts, 65 %). TET2 mut were strikingly overrepresentated compared to “non dmin” AML (19 %) (p<0.001) (Figure A) and often accompanied by trisomy 4, representing the most frequent chromosomal gain (12/76, 16 %). TP53 mut (24/75, 32 %) and U2AF1 mut (20/75, 27 %) were almost always mutually exclusive (except for 2 pts). While TP53 mut were strongly associated with ck (84 % vs. 6 % in non ck, p<0.001), U2AF1 mut were associated with APL-like cytomorphology (48 % vs. 5 % (1 case) in non APL-like cases, p=0.001). Both mut were highly overrepresented in MYC dmin pts compared to “non dmin” AML ( TP53: 32 % vs. 11 %, p<0.001; U2AF1: 27 % vs. 4 %, p<0.001) (Figure A). WGS analysis revealed an amplified chromosomal region with a size varying from 4.3 - 5.6 Mb and a commonly amplified region of 4.3 Mb (Chr 8:126,422,001-130,697,000). This region encompasses 6 protein coding genes ASAP1, CYRIB, GSMDC, LRATD2, POU5F1B and MYC as well as several non-coding RNAs including the long non-coding RNA PVT1. An effect of the amplified region on GE was confirmed by overexpression of MYC (p<0.001) and PVT1 (p<0.001) compared to “non dmin” AML (Figure B). The median overall survival of 41 evaluable pts was 16 months. Only TP53 mut was independently associated with inferior survival (HR: 11.0, p=0.001).
Conclusion: MN with MYC dmin are often AML-MR according to WHO 2022 definition, but even if not, show consistent features of severe dysplasia. They exhibit characteristic mutational patterns and distinct GE profiles, which are affected by a commonly amplified chromosomal region. Thus, we suggest MN with MYC dmin as a distinct genetically defined entity.
Disclosures
Summerer:MLL Munich Leukemia Laboratory: Current Employment. Walter:MLL Munich Leukemia Laboratory: Current Employment. Meggendorfer:MLL Munich Leukemia Laboratory: Current Employment. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Dicker:MLL Munich Leukemia Laboratory: Current Employment. Hutter:MLL Munich Leukemia Laboratory: Current Employment. Baer:MLL Munich Leukemia Laboratory: Current Employment. Stengel:MLL Munich Leukemia Laboratory: Current Employment. Kern:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Equity Ownership. Pohlkamp:MLL Munich Leukemia Laboratory: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal